A Comprehensive Overview of Tissue Engineering

Tissue engineering is a dynamic and
interdisciplinary field that merges principles from engineering, biology, and
medicine to develop biological substitutes that can restore, maintain, or
improve tissue function. It represents a paradigm shift in healthcare, offering
innovative solutions to address the challenges of tissue repair, regeneration,
and replacement. In this comprehensive report, we will delve deeply into the
foundational principles, diverse methodologies, cutting-edge applications,
persistent challenges, and future prospects of tissue engineering.
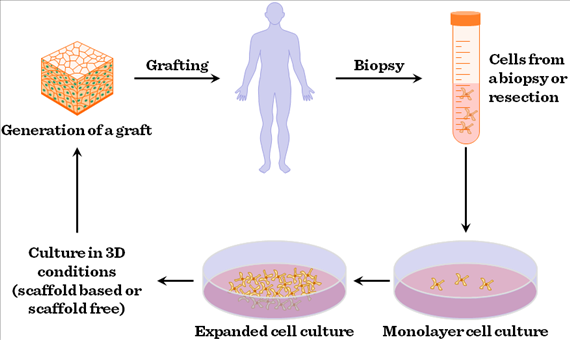
Basic principles of tissue engineering
Principles
of Tissue Engineering
At the heart of tissue engineering lies the concept of creating three-dimensional scaffolds that mimic the native extracellular matrix (ECM) of natural tissues. These scaffolds provide structural support and biochemical cues to guide cell attachment, proliferation, and differentiation. Biomaterials play a pivotal role in scaffold design, with a wide range of materials being utilized based on their biocompatibility, mechanical properties, and degradation kinetics. Natural polymers such as collagen, fibrin, and hyaluronic acid, as well as synthetic polymers like poly(lactic-co-glycolic acid) (PLGA) and polyethylene glycol (PEG), are commonly employed for scaffold fabrication.
In addition to scaffolds, cells are a
crucial component of tissue engineering constructs. Various cell sources,
including stem cells derived from bone marrow, adipose tissue, or umbilical
cord blood, as well as induced pluripotent stem cells (iPSCs), are utilized for
tissue regeneration. These cells are seeded onto the scaffolds and cultured
under controlled conditions, where they proliferate and differentiate into the
desired cell types. Growth factors, cytokines, and other biochemical stimuli
are often incorporated into the scaffold or delivered in the culture medium to
modulate cellular behavior and promote tissue-specific differentiation.
Methodologies
in Tissue Engineering
Tissue engineering encompasses a diverse array of methodologies tailored to specific tissue types and applications. Key methodologies include:
Biomaterials Selection: The choice of biomaterials is critical in scaffold design, with considerations including biocompatibility, mechanical properties, and degradation kinetics. Biomaterials can be categorized as natural, synthetic, or hybrid, each offering unique advantages and limitations. Natural biomaterials, such as collagen and alginate, provide a bioactive environment conducive to cell attachment and proliferation. Synthetic biomaterials, such as PLGA and PEG, offer tunable mechanical properties and degradation rates, allowing for precise control over scaffold characteristics.
Scaffold Fabrication: Various fabrication techniques are employed to create scaffolds with defined architecture and porosity. Common techniques include 3D printing, electrospinning, and freeze-drying, each offering unique advantages in terms of scalability, resolution, and material compatibility. 3D printing, or additive manufacturing, allows for the precise deposition of biomaterials layer by layer, enabling the fabrication of complex, patient-specific scaffolds. Electrospinning produces nanofibrous scaffolds with high surface area and porosity, mimicking the native ECM of tissues. Freeze-drying, or lyophilization, preserves the structural integrity of porous scaffolds while removing water content, facilitating cell infiltration and tissue integration.
Cell Sourcing and Culture: Cells are sourced from various tissues or differentiated from stem cells to populate tissue engineering constructs. Mesenchymal stem cells (MSCs) are commonly used for their multi-lineage differentiation potential and immunomodulatory properties. Endothelial cells, smooth muscle cells, and fibroblasts are also utilized to engineer vascularized tissues, such as blood vessels and heart muscle. Induced pluripotent stem cells (iPSCs), derived from adult somatic cells, offer a potentially limitless cell source for tissue regeneration. These cells are cultured in vitro under conditions that mimic the native tissue microenvironment, providing the necessary cues for cell proliferation, differentiation, and ECM deposition.
Biochemical Stimulation: Growth factors, cytokines, and other biochemical cues play a crucial role in regulating cellular behavior and tissue development. These bioactive molecules can be incorporated into the scaffold or delivered in the culture medium to mimic the native tissue microenvironment. For example, bone morphogenetic proteins (BMPs) are used to induce osteogenic differentiation in bone tissue engineering, while vascular endothelial growth factor (VEGF) promotes angiogenesis in vascular tissue engineering. Controlled release systems, such as microspheres or hydrogels, enable sustained delivery of growth factors, enhancing their bioactivity and efficacy in tissue regeneration.
Biomechanical
Stimulation: Mechanical forces exerted on cells and
tissues play a critical role in tissue development and maturation. Bioreactors
are utilized to provide controlled mechanical stimulation to cultured cells,
mimicking physiological conditions in vivo. Various types of bioreactors,
including perfusion systems, stretch chambers, and compression devices, can
apply mechanical forces such as fluid shear stress, cyclic strain, and
hydrostatic pressure to cultured cells. These biomechanical cues regulate cell
morphology, alignment, and ECM remodeling, ultimately influencing tissue
architecture and functionality.
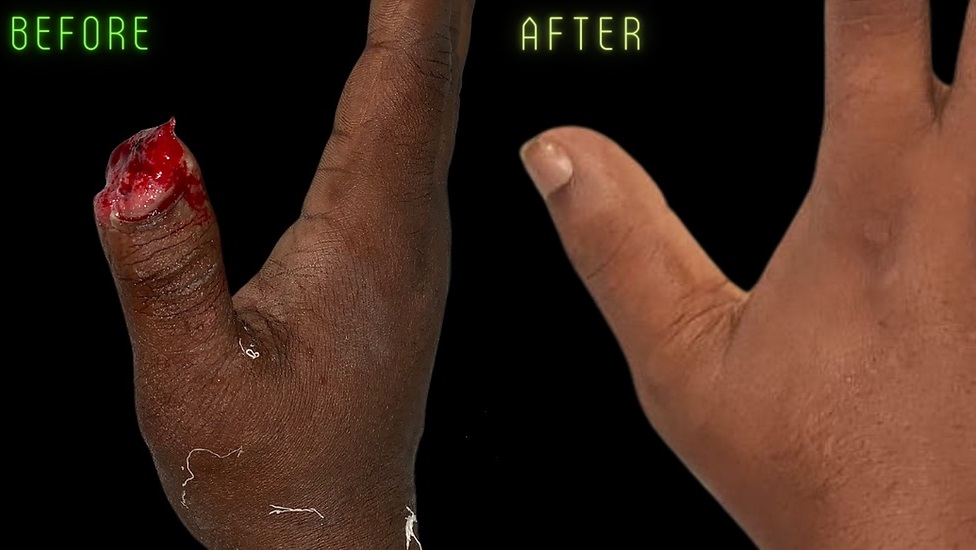
Applications
of Tissue Engineering
Tissue engineering has diverse applications across multiple domains of healthcare and biotechnology:
Regenerative Medicine: Engineered tissues and organs hold immense promise for regenerative medicine applications, including the repair and replacement of damaged or diseased tissues. Tissue-engineered skin substitutes have been successfully used to treat burns and chronic wounds, providing a scaffold for dermal regeneration and promoting wound healing. Engineered cartilage constructs, fabricated from chondrocytes seeded onto biodegradable scaffolds, have shown promise for treating cartilage defects and osteoarthritis. Tissue-engineered bone grafts, composed of osteogenic cells and bioactive ceramics, offer alternatives to autologous bone grafts for bone regeneration and fracture repair. Tissue-engineered organs, such as the bladder, trachea, and blood vessels, have been implanted in clinical trials, demonstrating the feasibility of organ transplantation using bioengineered constructs.
Disease Modeling: In vitro models of human tissues and organs are invaluable tools for studying disease mechanisms, screening drugs, and developing personalized therapies. Tissue-engineered models of cancer, cardiovascular diseases, neurodegenerative disorders, and other conditions provide physiologically relevant platforms for drug discovery and development. Organ-on-a-chip systems, comprising microfluidic devices seeded with tissue-specific cells, recapitulate key aspects of organ physiology and pathology, enabling high-throughput screening of drug candidates and personalized medicine approaches. These models offer insights into disease progression, drug response variability, and patient-specific treatment strategies, ultimately advancing precision medicine initiatives and improving clinical outcomes.
Drug Testing and Development: Tissue-engineered constructs are used to evaluate the safety, efficacy, and pharmacokinetics of pharmaceutical drugs. These models offer a more accurate representation of human physiology compared to traditional cell culture or animal models, leading to more reliable preclinical data and reduced drug development costs. Liver-on-a-chip devices, populated with hepatocytes and other liver-specific cell types, simulate drug metabolism, toxicity, and drug-drug interactions in vitro, providing valuable insights into drug-induced liver injury and pharmacokinetic variability. Kidney-on-a-chip models, consisting of proximal tubule epithelial cells and endothelial cells, replicate renal filtration and reabsorption processes, enabling nephrotoxicity screening and drug efficacy testing. These tissue-engineered platforms complement traditional preclinical models, accelerating the drug development pipeline and enhancing patient safety.
Cosmetic and Reconstructive Surgery: Tissue-engineered constructs are increasingly used in cosmetic and reconstructive procedures to enhance wound healing, tissue regeneration, and aesthetic outcomes. Engineered skin substitutes, composed of keratinocytes and fibroblasts seeded onto biocompatible scaffolds, promote re-epithelialization and collagen deposition, improving scar appearance and function. Tissue-engineered cartilage grafts, fabricated from chondrocytes and biomimetic scaffolds, offer alternatives to autologous cartilage grafts for nasal reconstruction and auricular augmentation. Breast implants composed of adipose-derived stem cells (ADSCs) encapsulated in hyaluronic acid hydrogels provide a minimally invasive option for breast augmentation and reconstruction. These tissue-engineered solutions address the limitations of traditional surgical techniques, offering patients natural-looking and long-lasting results.
Bioprinting: Three-dimensional bioprinting technology enables the precise
deposition of cells, biomaterials, and bioactive factors to create complex
tissues and organs with anatomical fidelity. Bioprinted tissues have
applications in regenerative medicine, drug screening, and personalized
medicine, paving the way for the fabrication of patient-specific implants and
organs-on-chips. Bioprinted skin substitutes, composed of keratinocytes and
fibroblasts encapsulated in bioinks, offer personalized wound care solutions
for patients with extensive burns or chronic wounds. Bioprinted vascular
constructs, seeded with endothelial cells and smooth muscle cells, facilitate
the fabrication of perfusable blood vessels for tissue regeneration and organ
transplantation. Organ-on-a-chip systems, fabricated using bioprinting
techniques, enable the integration of multiple tissue types into a single
platform, allowing for more complex and physiologically relevant disease models
and drug screening assays. These advances in bioprinting technology hold
promise for revolutionizing healthcare delivery and personalized medicine.
Challenges
and Future Directions
While tissue engineering has made significant strides, several challenges must be addressed to realize its full potential:
Vascularization: The development of functional vascular networks within engineered tissues is essential to ensure adequate nutrient and oxygen supply and promote tissue integration and maturation. Strategies such as prevascularization, angiogenic growth factors, and biomimetic scaffolds are being explored to address this challenge. Vascularization remains a significant hurdle in tissue engineering, particularly for large and complex tissues, such as solid organs.
Immunogenicity and Host Response: Minimizing immune rejection and inflammatory responses to implanted tissues remains a significant challenge in tissue engineering. Strategies to modulate the immune response, such as immune tolerance induction, surface modifications, and cell encapsulation, are under investigation to enhance the biocompatibility of engineered constructs. Immune rejection can compromise the long-term viability and functionality of tissue-engineered implants, highlighting the importance of immunomodulatory approaches in tissue engineering research and application.
Scale-Up and Commercialization: Scaling up tissue engineering processes for clinical translation and commercialization requires addressing regulatory, logistical, and economic challenges. Standardization of manufacturing techniques, quality control measures, and cost-effective production methods are essential to enable widespread adoption of tissue-engineered products. The transition from bench to bedside involves navigating complex regulatory pathways, securing funding, and establishing partnerships with industry stakeholders, highlighting the need for interdisciplinary collaboration and strategic planning in tissue engineering endeavors.
Integration with Host Tissues: Achieving seamless integration between engineered tissues and host tissues is critical for long-term functionality and patient outcomes. Strategies to promote host tissue ingrowth, remodeling, and vascularization are being pursued to enhance the integration and survival of implanted constructs. Tissue-engineered implants must exhibit biocompatibility, stability, and functionality in vivo to avoid complications such as fibrosis, implant rejection, and infection. Biomaterials with tunable properties and bioactive coatings are being developed to facilitate tissue integration and improve implant performance.
Bioethical
Considerations: Ethical considerations surrounding
the use of stem cells, genetic engineering, and organ transplantation must be
carefully addressed to ensure responsible innovation and equitable access to
tissue engineering technologies. Ethical guidelines, informed consent
procedures, and stakeholder engagement are essential for navigating the complex
ethical landscape of tissue engineering research and application. The use of
human embryonic stem cells (hESCs) and genome editing technologies, such as
CRISPR-Cas9, raise ethical concerns related to patient safety, privacy, and
social justice, underscoring the importance of ethical reflection and community
engagement in tissue engineering endeavors.
Editor’s
Thoughts:
Tissue engineering holds tremendous promise
for revolutionizing healthcare delivery and personalized medicine. By
leveraging advances in biomaterials, cell biology, and biomanufacturing
technologies, tissue engineers are poised to address unmet medical needs and
improve patient outcomes across a wide range of applications. Despite the
remaining challenges, including vascularization, immunogenicity, scale-up,
integration, and ethics, the future of tissue engineering is bright, with the
potential to transform medicine and enhance the quality of life for millions of
people worldwide.


